Drugs and Medications
Is Ozempic Good For Insulin Resistance
Ozempic, commonly known as semaglutide, is a medicine licensed by the FDA to treat type 2 diabetes. It is a glucagon-like peptide-1 (GLP-1) receptor agonist that stimulates insulin secretion while inhibiting glucagon secretion. However, there is significant dispute about whether Ozempic is effective in treating insulin resistance, a major consequence of type 2 diabetes.
Insulin resistance occurs when cells in the body become resistant to insulin, resulting in elevated blood sugar levels. Ozempic has been demonstrated to enhance blood sugar control in persons with type 2 diabetes, although its effects on insulin resistance are unclear. Some research indicate that Ozempic may increase insulin sensitivity and reduce insulin resistance, while others showed no meaningful impact.
Despite the mixed findings, many healthcare practitioners continue to recommend Ozempic for the treatment of type 2 diabetes because it has been demonstrated to improve blood sugar control and lower the risk of cardiovascular events. However, further research is needed to evaluate whether Ozempic is a useful alternative for persons with insulin resistance in particular.
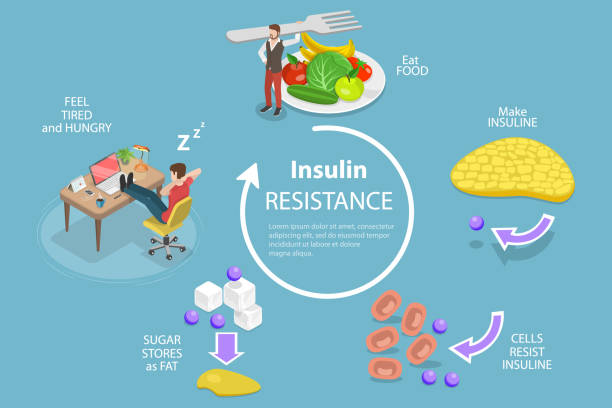
Understanding Insulin Resistance and Type 2 Diabetes
Insulin is a pancreatic hormone that helps regulate blood glucose levels. The body’s cells rely primarily on glucose for energy. When glucose levels in the blood rise, the pancreas secretes insulin, which instructs cells to absorb glucose and convert it to energy.
Role of Insulin and Glucose
Insulin is essential for keeping blood sugar levels under control. When insulin is operating properly, glucose can enter cells and be used for energy. However, when insulin levels are too low or the body becomes resistant to insulin, glucose can accumulate in the bloodstream, resulting in hyperglycemia.
Development of Insulin Resistance
Insulin resistance develops when the body’s cells become less sensitive to insulin signals, making it difficult for glucose to enter. As a result, the pancreas must produce more insulin to keep blood sugar levels under control, resulting in greater insulin levels in the bloodstream. Over time, this can cause pancreatic exhaustion, and the pancreas may no longer be able to generate enough insulin to keep blood sugar levels within acceptable limits.
Implications for Type 2 Diabetes
Insulin resistance is a major risk factor for developing type II diabetes. In persons with type 2 diabetes, the pancreas generates insulin, but the body’s cells become resistant to its signals, resulting in chronically high blood sugar. This can harm organs and tissues throughout the body, resulting in consequences like cardiovascular disease, kidney damage, and nerve damage.
In summary, insulin resistance is a key factor in the development of type 2 diabetes. Insulin resistance impairs the body’s capacity to control blood sugar levels, resulting in hyperglycemia and the development of chronic health disorders.

Ozempic and Its Mechanism of Action
Ozempic, a GLP-1 receptor agonist, is FDA-approved for the treatment of type 2 diabetes. Ozempic’s active ingredient is semaglutide, which mimics the actions of GLP-1, a hormone that the body naturally produces.
GLP-1 Receptor Agonist Explained
GLP-1 receptor agonists, such as Ozempic, bind to GLP-1 receptors in the body, which are predominantly found in the pancreas. When GLP-1 binds to these receptors, it increases the release of insulin, lowering blood sugar levels. GLP-1 also delays stomach emptying, which can help suppress hunger and promote weight loss.
Effects on Blood Sugar and Insulin Secretion
Studies have indicated that Ozempic is effective for lowering A1C readings, which are average blood sugar levels throughout time. In clinical trials, patients using Ozempic had significantly lower A1C levels than those taking a placebo. Ozempic also promotes insulin secretion, which can further reduce blood sugar levels.
Impact on Weight and Appetite
Ozempic has been demonstrated to effectively promote weight loss in type 2 diabetic patients. In clinical trials, participants taking Ozempic lost an average of 5-10% of their body weight. This is most likely owing to the fact that GLP-1 receptor agonists, such as Ozempic, decrease stomach emptying, which can suppress appetite and increase feelings of fullness.
However, like many drugs, Ozempic might produce negative effects. Ozempic’s most common side effects are stomach upset, nausea, vomiting, diarrhea, and constipation. In rare situations, Ozempic may raise the risk of pancreatitis, a dangerous illness requiring rapid medical intervention.
Ozempic is given as a subcutaneous injection once a week. The recommended first dosage is 0.25 mg once per week, which can be increased to 0.5 mg once per week after 4 weeks. Ozempic should not be combined with glimepiride or any other insulin-stimulating medicines.
Overall, Ozempic is a viable therapeutic choice for type 2 diabetes patients who are having difficulty with insulin resistance, weight management, and hunger control.

Clinical Evidence and FDA Approval
Key Findings from Clinical Trials
Ozempic (semaglutide) is a once-weekly injectable drug approved by the FDA for the treatment of type 2 diabetes in adults. It belongs to a class of drugs known as glucagon-like peptide-1 (GLP-1) receptor agonists, which function by increasing insulin production, lowering glucagon secretion, and delaying gastric emptying.
Several clinical trials have been done to assess Ozempic’s efficacy and safety in the treatment of type 2 diabetes and its consequences. The SUSTAIN clinical trial program, which included over 8,000 patients, found that Ozempic was more effective than other GLP-1 receptor agonists and placebo in lowering HbA1c levels and body weight while also improving cardiovascular outcomes.
Another clinical trial, PIONEER 6, looked at Ozempic’s cardiovascular safety in patients with T2DM, established cardiovascular illness, or high cardiovascular risk. The experiment discovered that Ozempic lowered the risk of significant adverse cardiovascular events compared to placebo.
FDA Approval and Off-Label Uses
The Food and Drug Administration (FDA) approved Ozempic in December 2017 for the treatment of T2DM in adults. It has not been licensed for use in children or in the treatment of type 1 diabetes.
Ozempic is only FDA-approved for the treatment of type 2 diabetes, however it may have off-label use. GLP-1 receptor agonists, such as Ozempic, have been shown in some studies to be beneficial in the treatment of obesity and insulin resistance. However, additional research is required to corroborate these conclusions.
Ozempic, like many drugs, can produce negative effects. The most common adverse effects include nausea, vomiting, diarrhea, and stomach pain. In rare situations, Ozempic might cause anaphylaxis or thyroid C-cell tumors. Ozempic may interact with other drugs, including sulfonylureas and metformin.
Overall, clinical evidence suggests that Ozempic is an effective and safe medication for individuals with type 2 diabetes. Patients should, however, consult with their healthcare professional about Ozempic’s potential advantages and hazards, as well as if it is the best therapy option for them.
Safety Profile and Considerations
Common and Serious Side Effects
Ozempic is generally well tolerated, although like all medications, it might produce negative effects. The most prevalent side effects documented in clinical trials were nausea, vomiting, diarrhea, constipation, and injection site reactions. These adverse effects are typically minimal and resolve on their own. Patients should seek medical attention if their symptoms persist or worsen.
In rare situations, Ozempic can produce serious adverse effects such as pancreatitis, allergic responses, thyroid tumors, and renal damage. Patients should seek medical assistance right once if they feel severe stomach pain, shortness of breath, edema, or mood swings.
Contraindications and Precautions
Ozempic should not be used if you have a history of hypersensitivity to semaglutide or any of its components. It should also not be used in people with a personal or family history of medullary thyroid cancer (MTC), as well as those with multiple endocrine neoplasia syndrome type 2.
Patients with a history of pancreatitis or alcohol misuse should exercise caution when using Ozempic. Ozempic may cause hypoglycemia when used with sulfonylureas, hence patients should be continuously watched for low blood sugar levels.
Ozempic has been demonstrated to minimize cardiovascular events in patients with type 2 diabetes and preexisting cardiovascular disease. However, it should not be used to replace effective care of cardiovascular risk factors such as blood pressure and cholesterol.
To summarize, Ozempic is a safe and effective medication for insulin resistance when administered correctly. Patients should be informed of the potential adverse effects and contraindications and should contact their doctor if they have any concerns.
Conclusion
In conclusion, growing evidence suggests that Ozempic may be a helpful alternative for treating insulin resistance in people with type 2 diabetes. Aside from its principal role in improving glycemic control, Ozempic’s impact on weight management and potential cardiovascular benefits could help to develop a more holistic strategy to insulin resistance. While further research is needed to determine the full degree of Ozempic’s effects on insulin resistance, preliminary data suggest that it could be a useful treatment tool. As with any medical decision, individuals should contact with their healthcare providers to identify the best treatment strategy for their personal health profile and needs. Ozempic is a hopeful step forward in the pursuit of efficient insulin resistance treatments in diabetes care.
Journey of self discovery


